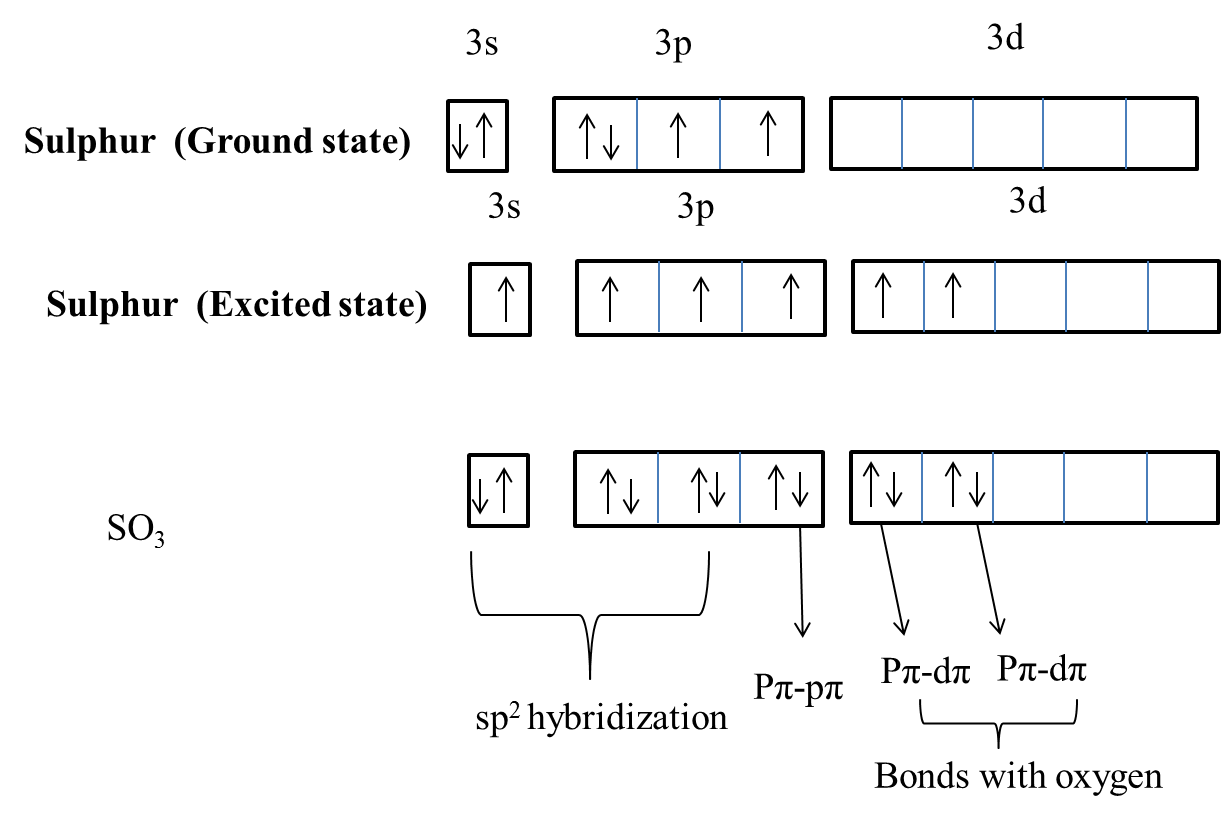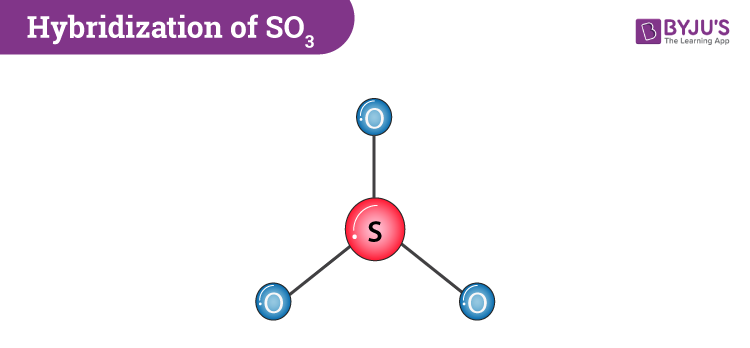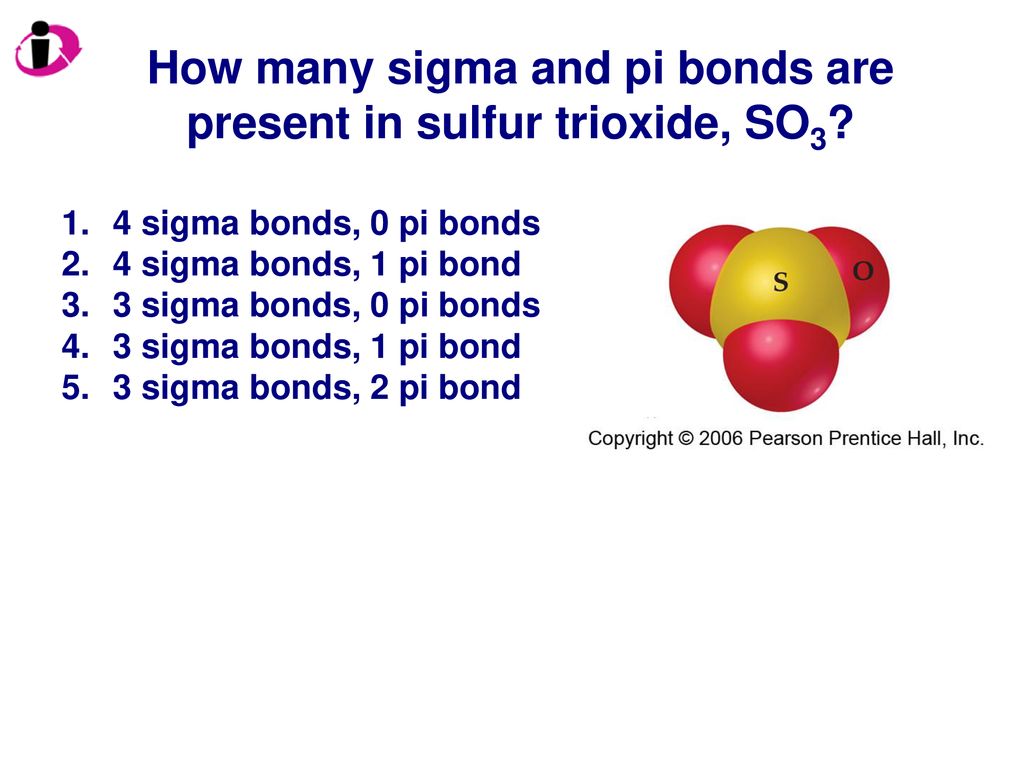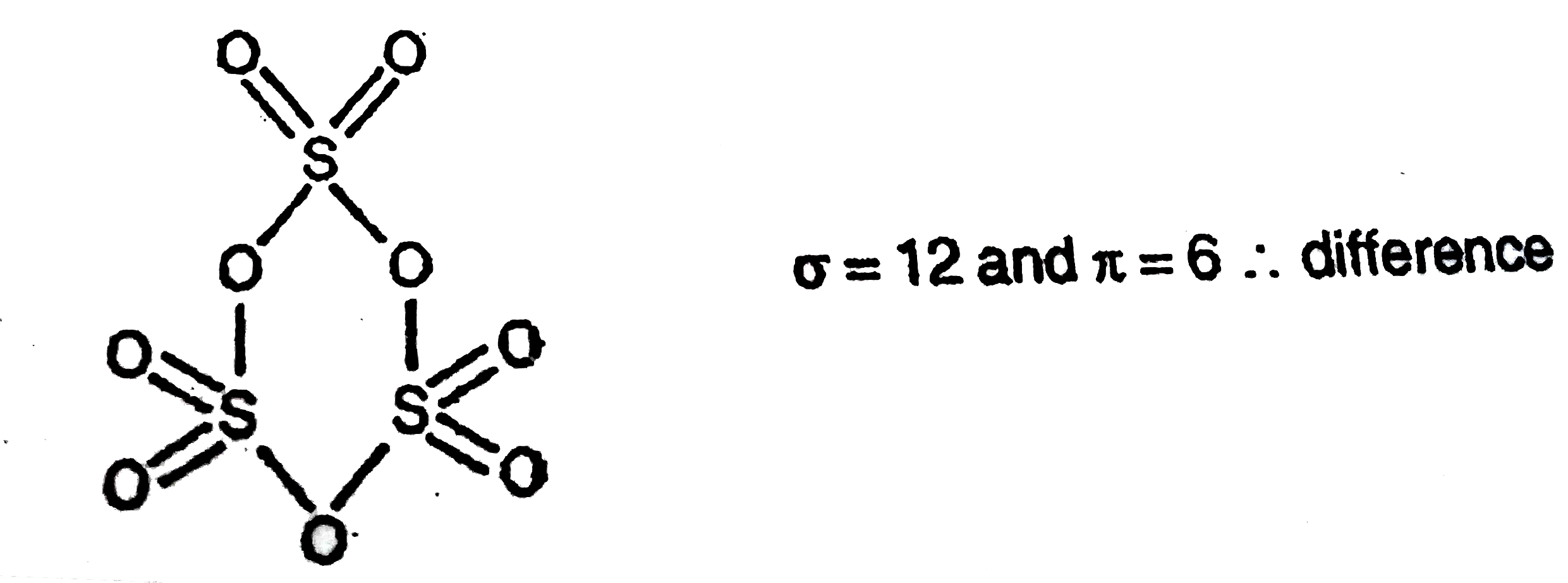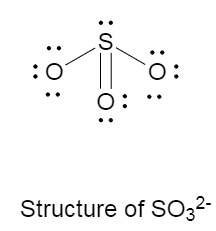
In $SO_3^{2 - }$ :A.$d\\pi - p\\pi $ bond between $S$ and $O$ is delocalized.B.Bonds between $S$ and $O$ are equivalent.C.There is $s{p^3}$ hybridized sulphur atomD.All of the facts given above are
![Describe the bonding in SO2 and SO3 using the localized electron model (hybrid orbital theory). How would the molecular orbital model describe the [{MathJax fullWidth='false' \pi }] bonding in these two compounds? Describe the bonding in SO2 and SO3 using the localized electron model (hybrid orbital theory). How would the molecular orbital model describe the [{MathJax fullWidth='false' \pi }] bonding in these two compounds?](https://homework.study.com/cimages/multimages/16/screenshot_2022-12-02_1257511014901097068014803.png)
Describe the bonding in SO2 and SO3 using the localized electron model (hybrid orbital theory). How would the molecular orbital model describe the [{MathJax fullWidth='false' \pi }] bonding in these two compounds?

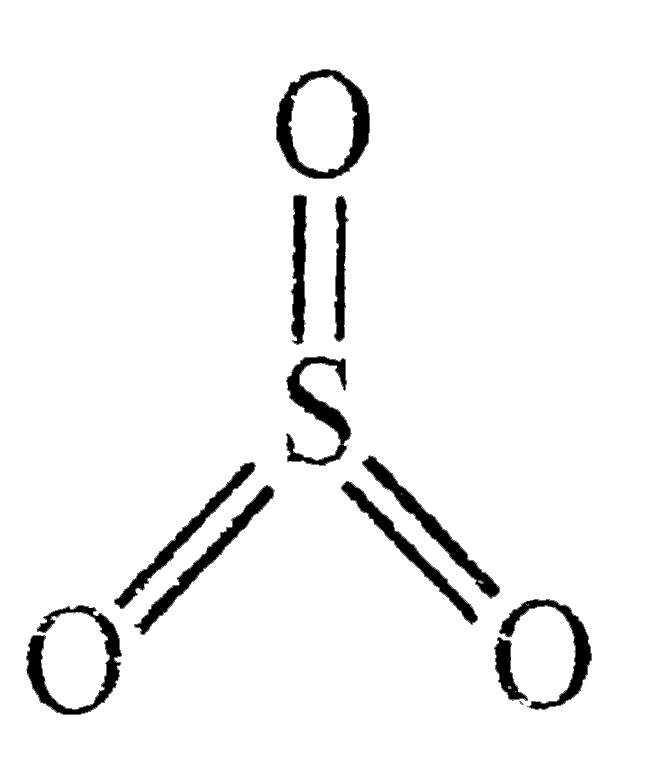
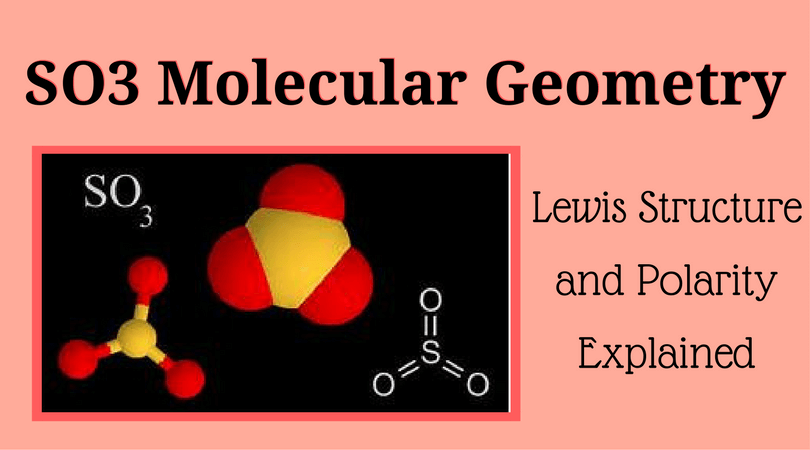
In the LEWIS Structure of SO3, the number of sigma and pi bonds respectively are 3, 3 3, 2 - Chemistry - The Solid State - 14954939 | Meritnation.com