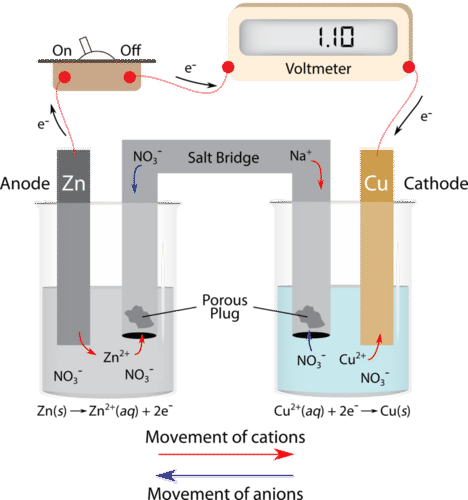
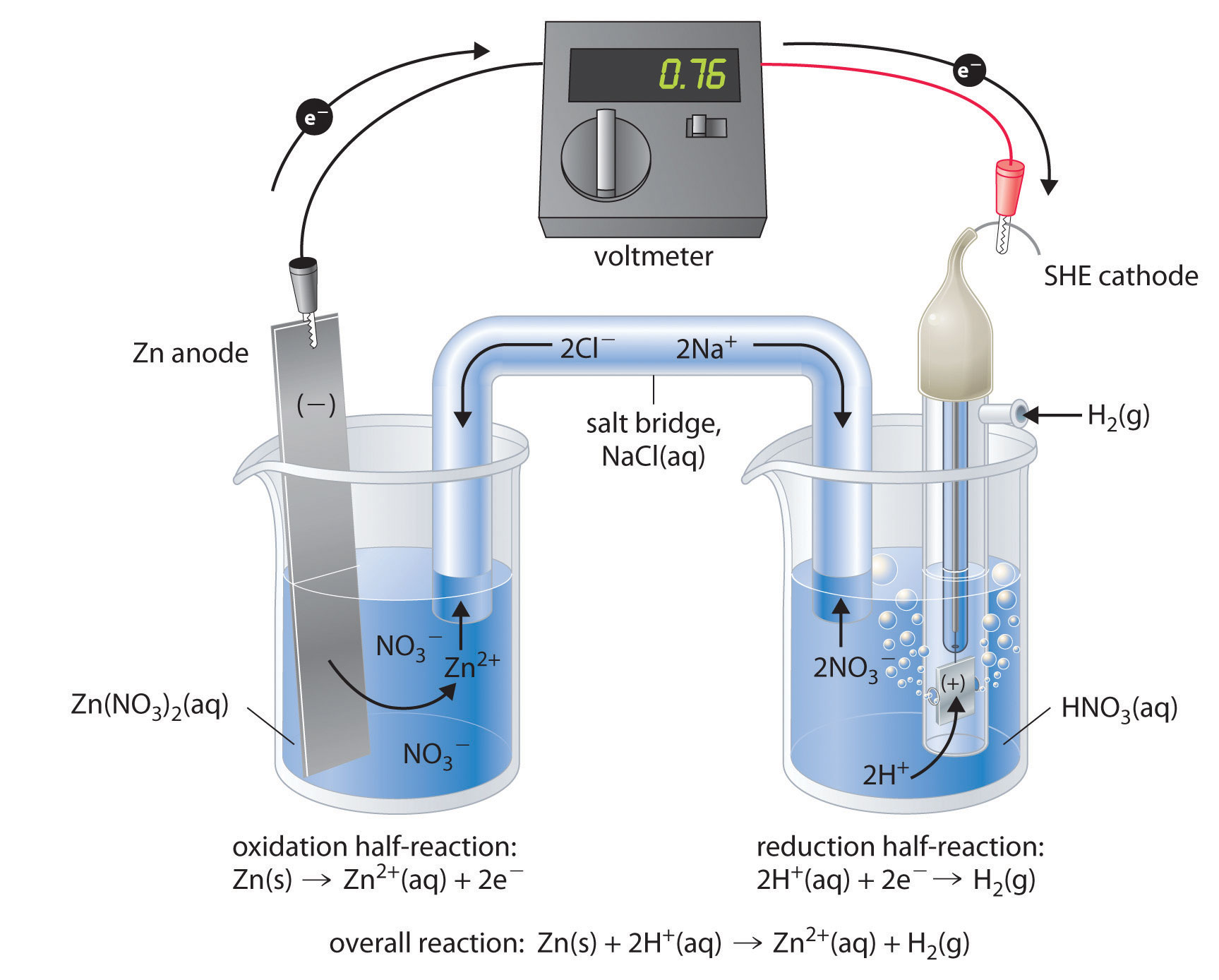
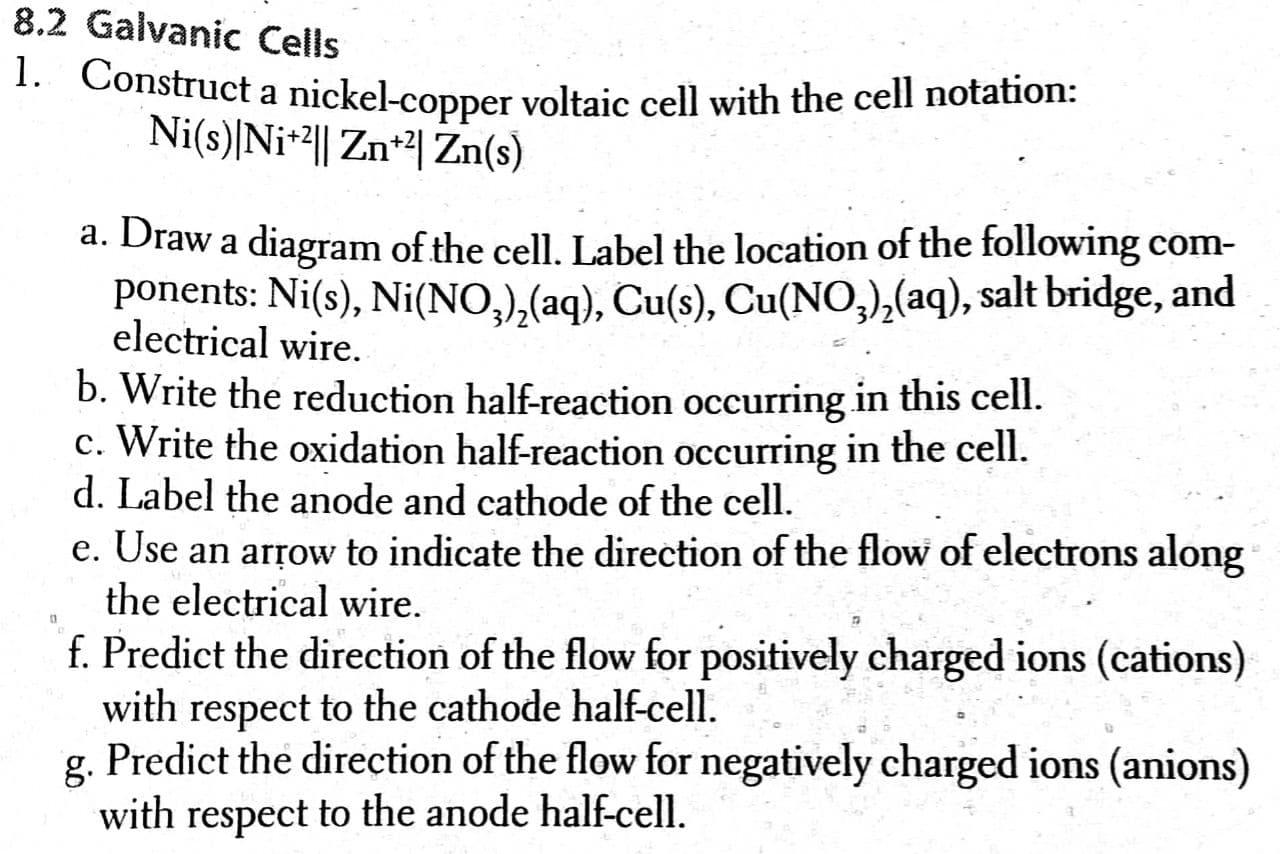
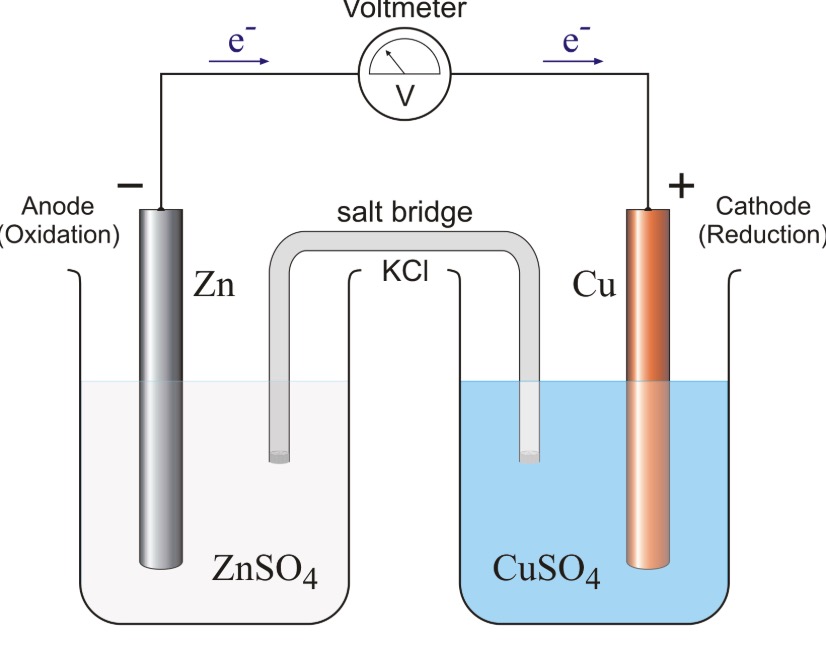
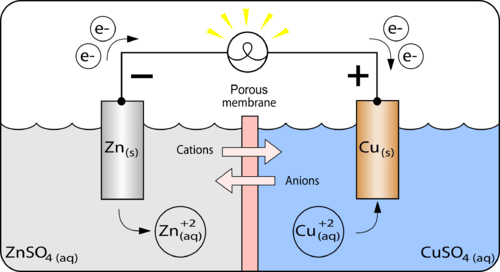
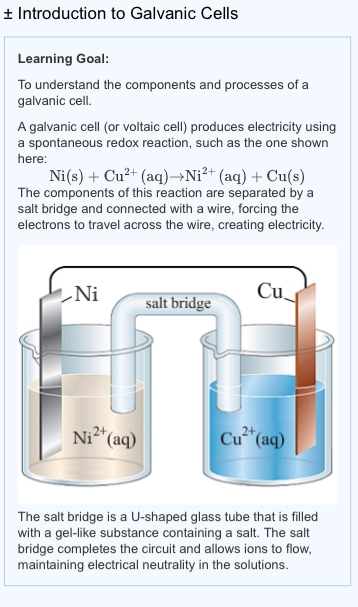
Mental model drawing for a given galvanic cell (Zn|Zn 2+ ||Cu|Cu 2+ ,... | Download Scientific Diagram

A galvanic cell is made up of a copper electrode in a 1.0 M copper (II) sulfate solution, a silver electrode in a 1.0 M silver nitrate solution, and a salt bridge

Draw a picture of a voltaic electrochemical cell using Ni and Fe electrodes and 0.1 M NiCl_2 and FeCl_2 solutions. Be sure to label the following: anode, cathode, salt bridge, the metals,

Sketch a voltaic cell for this redox reaction: Ni^{2+} (aq) + Mg (s) to Ni (s) + Mg^{2 +}(aq) a. Label the anode and cathode. b. Write the half reactions. c. Indicate

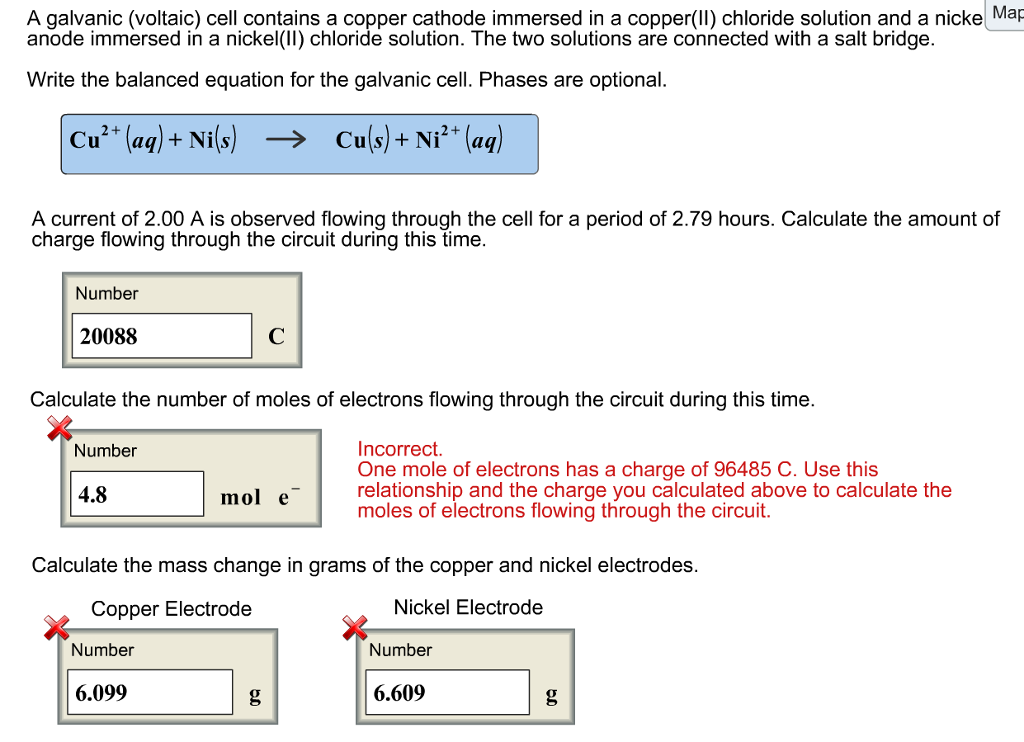
SOLVED: Suppose the galvanic cell sketched below is powered by the following reaction: Ni(s)-+CuSO 4(aq) NiSO4(aq)+Cu(s) S1 S2 Write balanced equation for the half- reaction that happens at the cathode of this

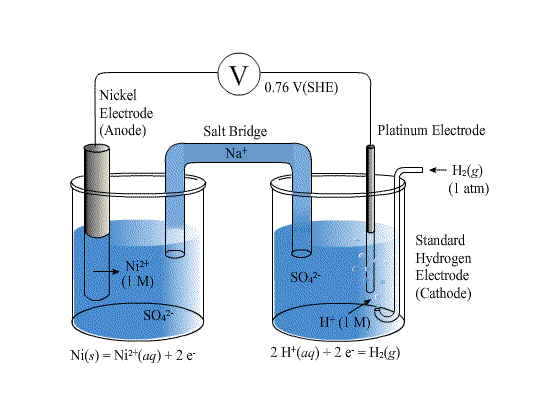
The EMF of the cell Ni `|Ni|Ni^(2+) ||Cu^(2+) |Cu(s)` is `0.59 ` volt. The standard reduction - YouTube

A voltaic cell consists of a silver-silver ion half-cell and a nickel-nickel(II) ion half-cell. Silver ion is reduced during operation of the cell. Sketch the cell, labeling the anode and cathode and